Lithium is an extremely reactive metal, meaning that, although it does exist in its metallic form, it is exceedingly rare to find it in nature. Like other alkali metals, lithium is prone to forming compounds with other elements: lithium carbonate, Li2CO3, is one of the most common and economically important lithium-bearing minerals. For this reason, lithium demand is often expressed as Lithium Carbonate Equivalent, or LCE. LCE is used to standardize the trade and measurement of various lithium compounds against one common reference.
For example, if a lithium mine produces lithium as a different compound (like lithium hydroxide), they might report production figures in terms of LCE to help buyers, investors, and regulators understand the amount of lithium being produced in terms that are comparable across different forms of the metal. This is particularly important for pricing and market analysis, as it provides a uniform basis for evaluating the lithium content of various sources and processed materials in the supply chain.
The conversion rate between lithium and lithium carbonate depends of course on their corresponding molar masses:
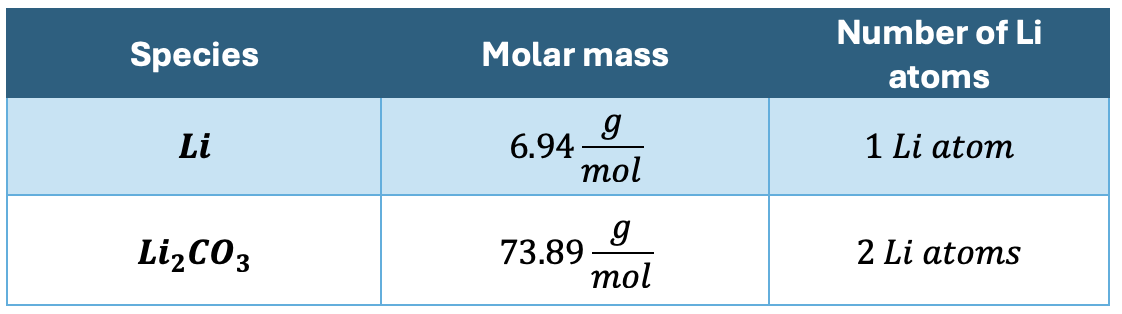
The conversion factor is therefore the following:
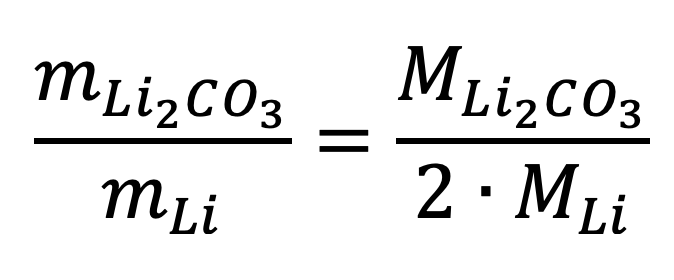
Given the established molar masses for each compound, the value of the conversion factor is the following:
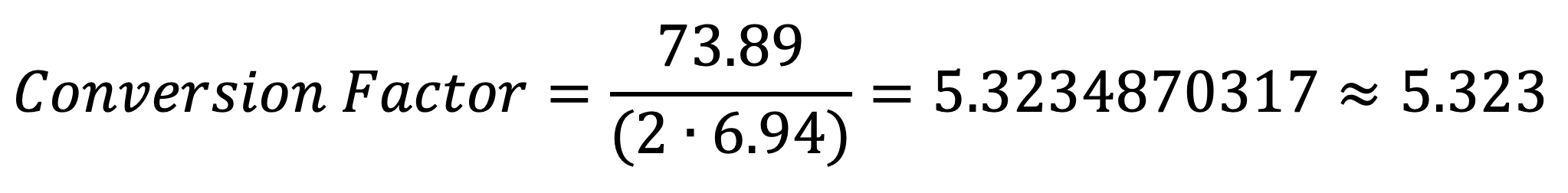
This value indicates the amount of lithium carbonate that is required from a certain amount of lithium. Because lithium carbonate only has approximately 18.8% of lithium by weight, more lithium carbonate would be needed to obtain the same amount of lithium than would be obtained from the pure metal. The numbers in the following table provide a clearer example: For every 7.2 mass units of lithium (be it in grams or kilograms) needed for the NCA cathode, the equivalent amount of lithium carbonate that is required is 38. This number was obtained with the previous formula:
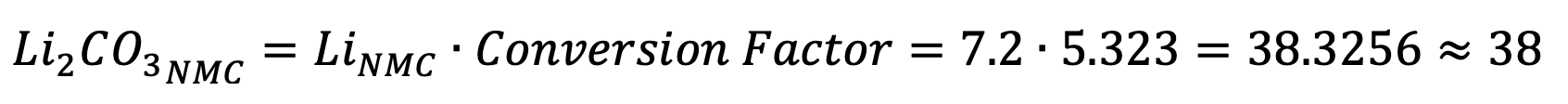
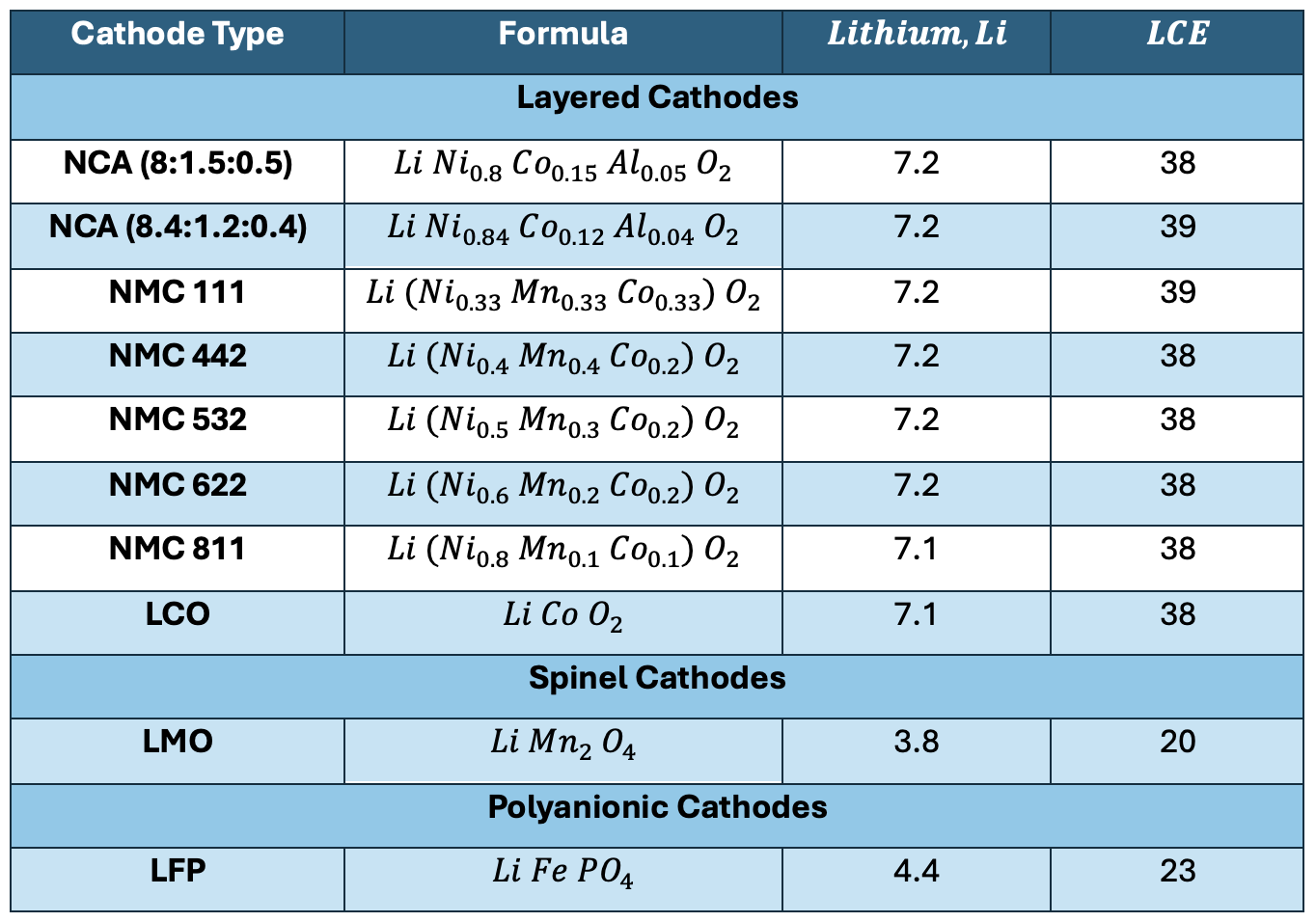
As the previous table demonstrates, all layered, nickel-manganese mixtures require almost twice the amount of lithium (and lithium carbonate) as iron-based cathodes, which are exclusively polyanionic. It is interesting to note that the spinel LMO cathode requires the least amount of lithium.