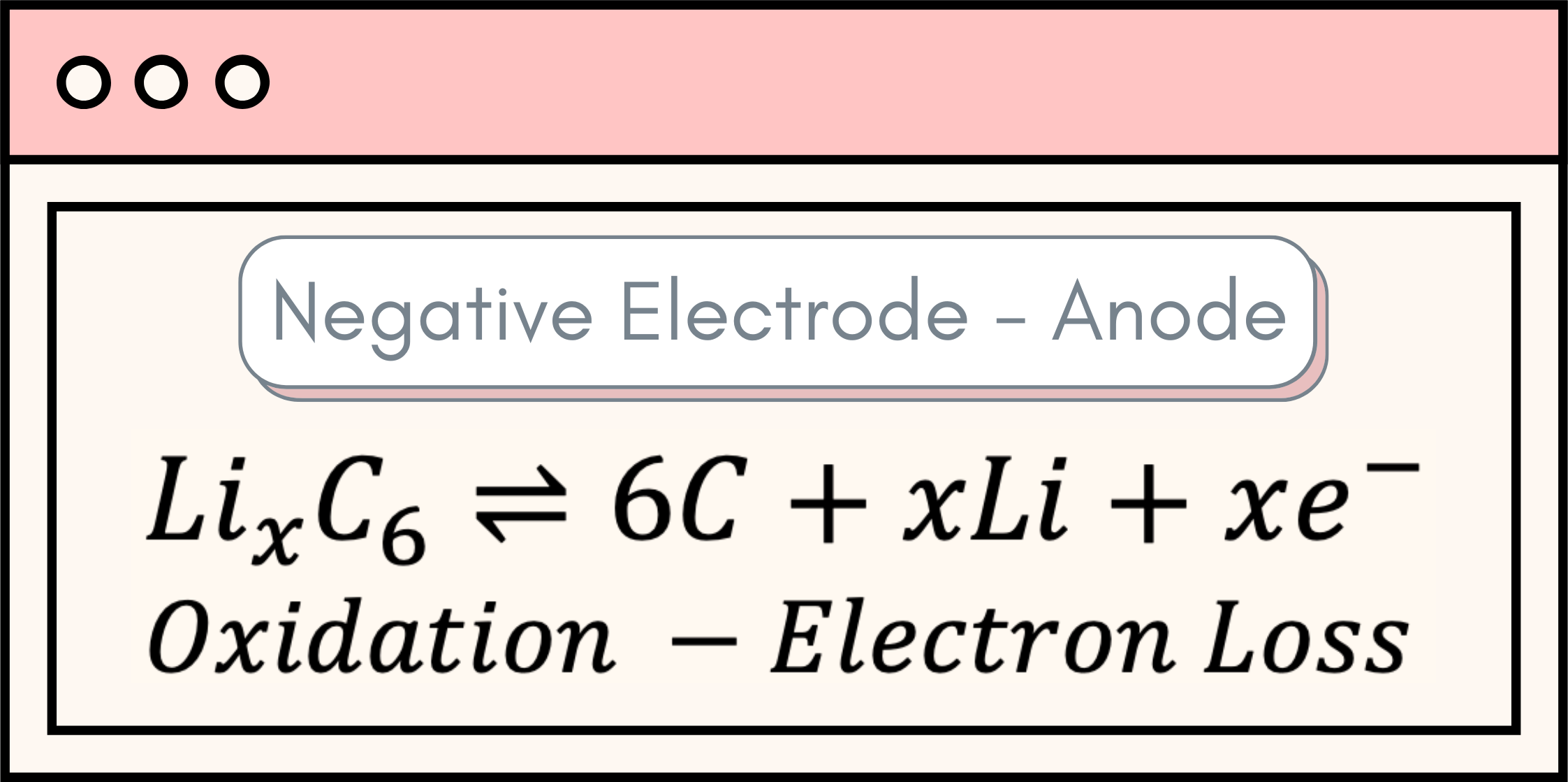
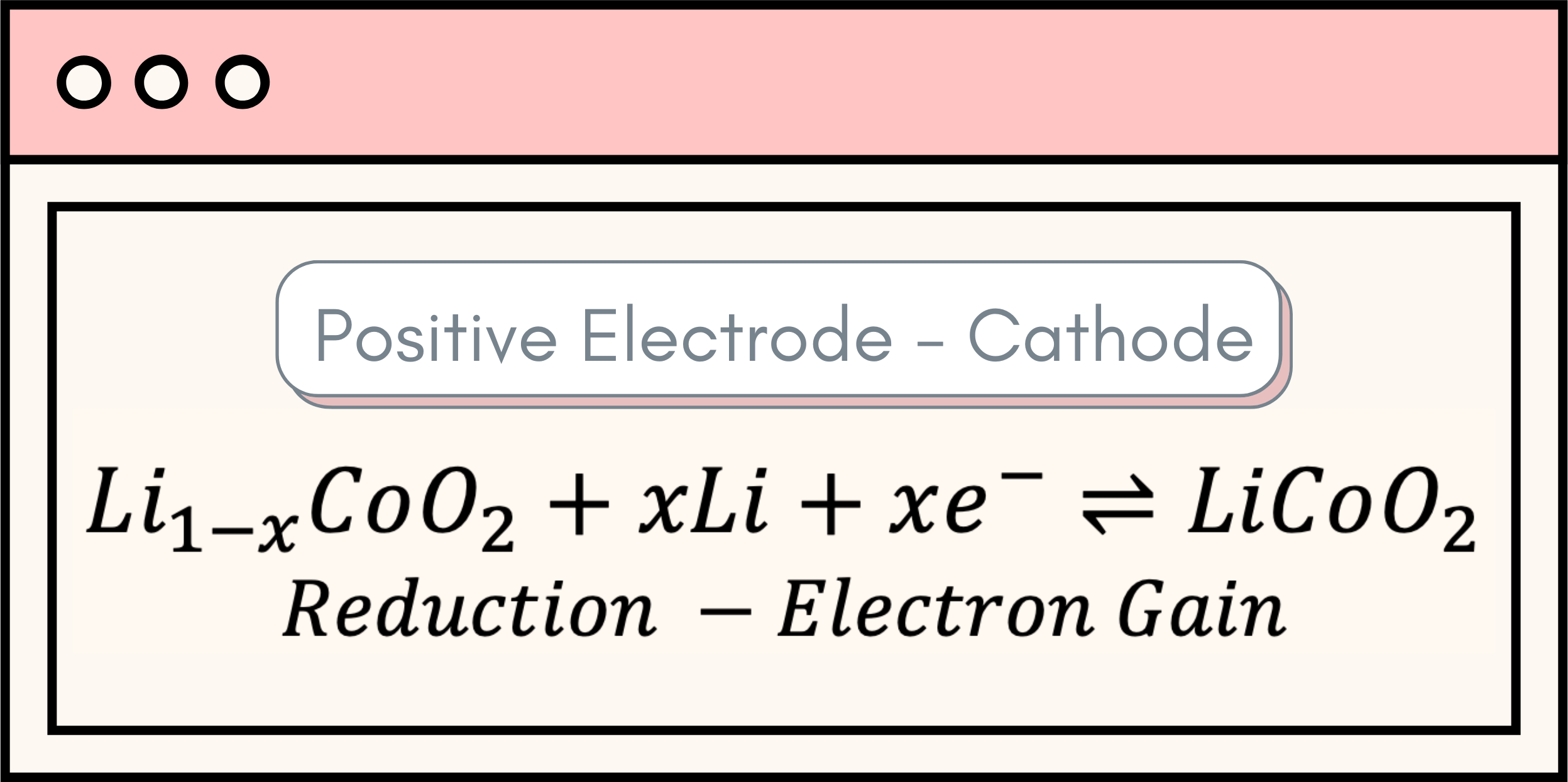
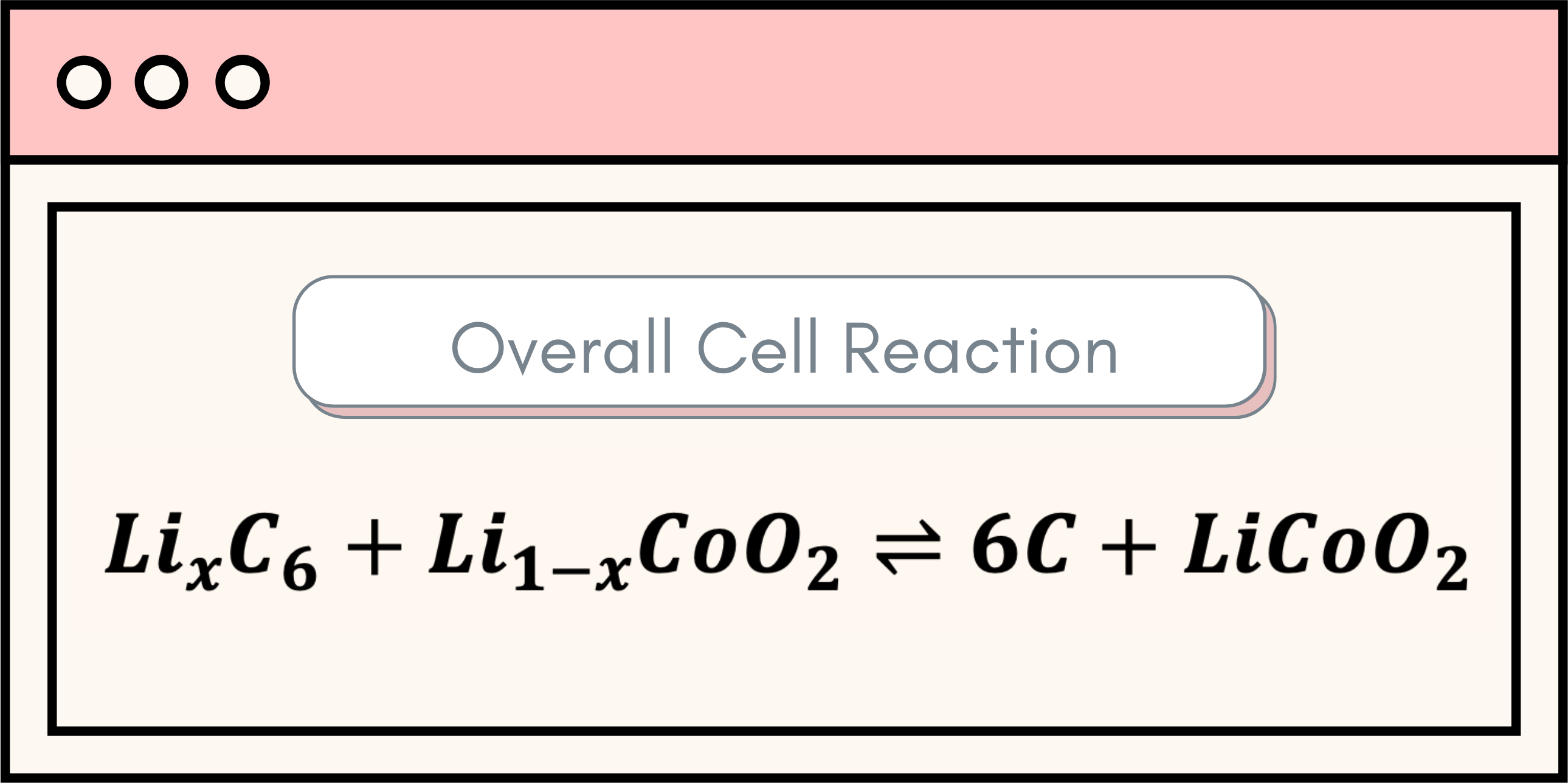
There is a long list of available battery technologies, but the core of a battery’s operation resides in three main components: the positive electrode, the negative electrode, and the electrolyte. The heart of a battery lies in its ability to produce electrical energy through a series of chemical reactions, known as oxidation and reduction – commonly abbreviated as REDOX reactions. Oxidation refers to a process where an atom loses electrons, while reduction denotes an atom gaining electrons. Indeed, these two processes occur at the bulk of the battery electrodes, with oxidation taking place in the negative electrode and reduction transpiring in the positive electrode. These reactions can be observed below:
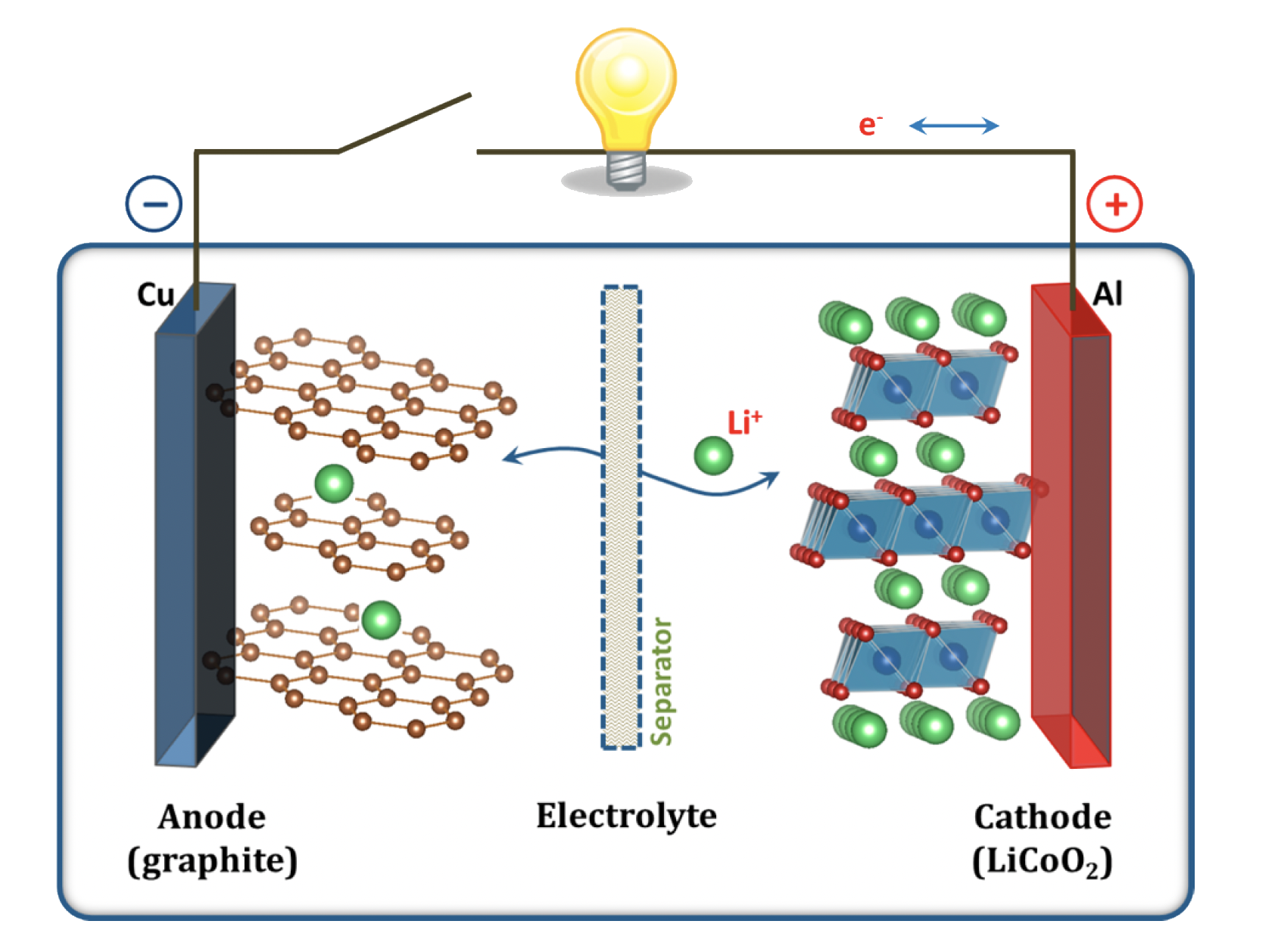
When a battery is discharging – or delivering energy – positive ions detach from the negative electrode and travel through the electrolyte medium towards the positive electrode. The electrons travel in the same direction, for they are magnetically attracted to the positive ions. This ubiquitous electronic and ionic journey is what powers our everyday devices: the electricity that is generated does work on the system by moving electric charges through an external circuit. This process is spontaneous, meaning it has a natural tendency to occur without the need for an external source of energy input.
When all the positive ions have relinquished the negative electrode and are now intercalated in the positive one, the battery is considered to be discharged, and must now undergo a charging process to be able to perform work again. For this to happen, an external, electric current is run through the battery, which detaches the positive ions from the positive electrode and moves them back into the negative one. It then becomes evident that this process is non-spontaneous, for it does not transpire naturally but rather, requires external energy to take place. The following diagram demonstrates this process in action, with the lithium ions moving inside each electrode during the cycling of the battery.